AI-based knowledge management in the life sciences
How companies can get the most out of expertise generated from existing data – from food and drug research and development to regulatory approval.
Pharmaceutical and life science companies are staring down new challenges in the wake of the digital transformation. Particularly in a sector that relies so heavily on the availability of expertise in the form of data, intelligent knowledge management is taking on a steadily more pervasive role. Find out how insight engines can help companies and government agencies streamline and accelerate research and regulatory processes.
- Proactive information provision in research and development
Innovation is the only route to advancements in medicine. Medical research is the vehicle that allows patients to benefit from new and innovative treatment options. Particularly in the field of R&D, data plays an extremely important role. Studies, results from trials, information on active ingredients or experiments, expert opinions – pharmaceutical companies have access to enormous and ever-increasing amounts of data. The only way to effectively accelerate innovation is by providing targeted and efficient access to the information that is needed.
By recognizing patterns and understanding content semantically, insight engines deliver information about scientific papers, reports, and studies at the touch of a button, and also allow companies to locate the relevant experts in a given field more efficiently.
- Accelerating regulatory approval in the pharmaceutical environment
New drugs have to undergo extensive approval procedures. The process is dominated by the obligation to verify the nature, quality, and purity of the drug. Every approval involves a tremendous administrative outlay. Several thousand different documents, such as studies, tests, and reports, have to be submitted from multiple disparate data sources. Insight engines deliver a welcome solution to this challenge by leveraging powerful AI applications like deep learning to dramatically accelerate and optimize the supply, analysis, linkage, and interpretation of information from different applications.
- Streamlining the process of regulatory drug approval and marketing authorizations
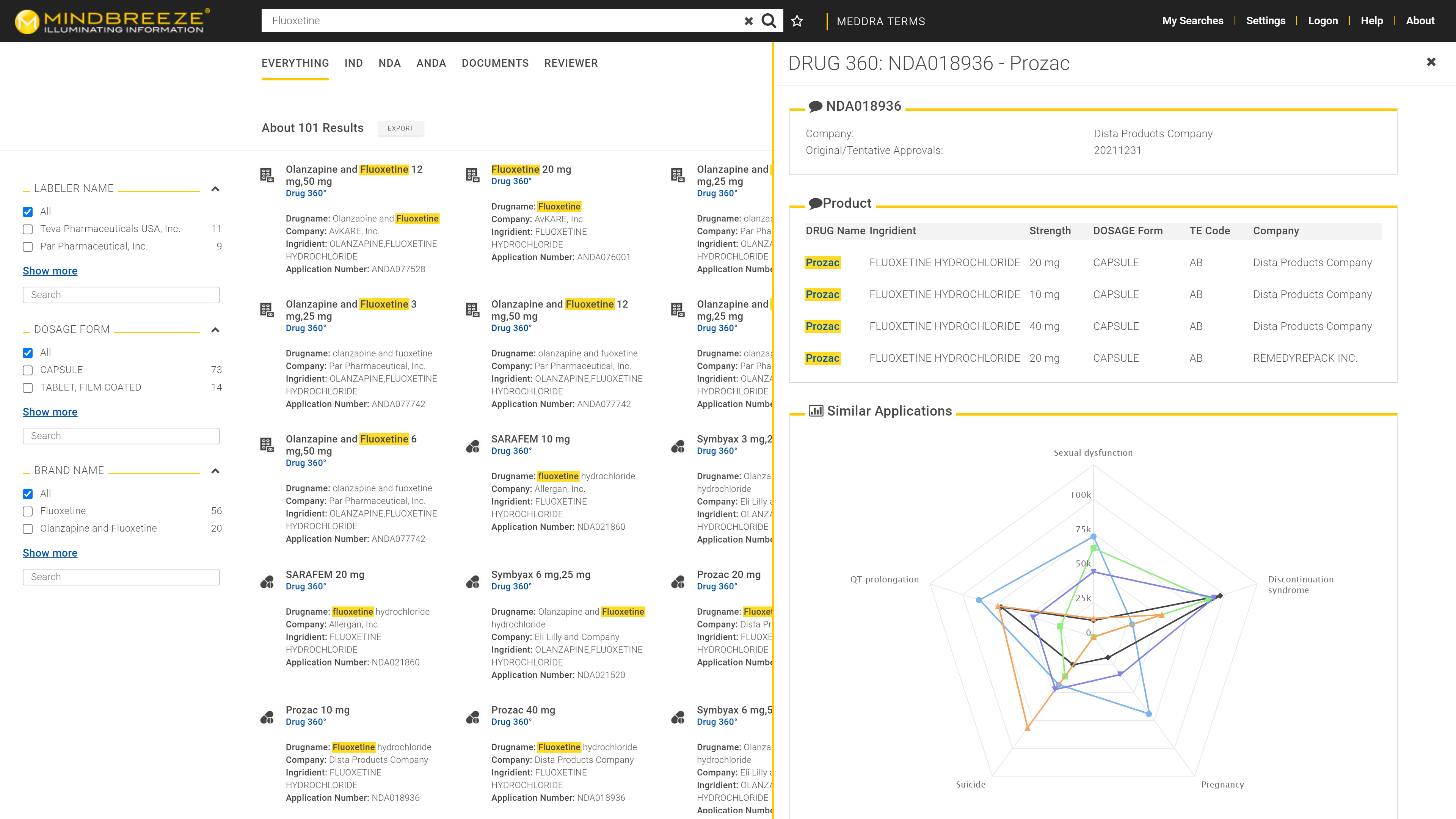
Regulatory approval for medicines, food, and active ingredients is contingent upon the submission of thousands of documents. The same holds true for new registrations, updates, and changes in formulations. The task of the regulatory authority is to examine whether a submission satisfies the requirements and whether the drugs can be approved. This process draws on a vast array of internal and external data. In order to mitigate the risk of legal disputes following approval, it is critical for reviewers to make informed and, most importantly, consistent decisions. With the aid of AI, this amassment of data can be indexed and made available to the reviewers, who then have a 360-degree view of any given submission, drug, food, or active ingredient seeking approval – a marked improvement in terms of transparency, safety, and workflow.
- Invaluable research tool for inspections and audits
The Medicines Act stipulates that all companies and facilities in which medicinal products are manufactured, tested, stored, packaged, traded, or placed on the market are subject to regular inspections by the authorities. Likewise, independent audits are conducted to monitor compliance with the specifications and the practical implementation of certain standards.
Inspections and independent audits have inevitably forced companies to invest countless hours, often at very short notice, to compile the necessary documentation. Empowered by the capability to integrate every data source and format, generate cross-references to different catalogs, and understand the content semantically, AI-based knowledge management solutions simplify this process dramatically.
 Find out how this works in practice in our case study
Find out how this works in practice in our case study
Open Case Study
Latest Blogs
The New Collaborative Era: Humans + AI in 2026
Moving Beyond the Automation Fear NarrativeFor years, public debate around AI focused on a single question: Will machines replace human workers?But in 2026, that narrative is no longer relevant.
Why AI Trust Will Define Enterprise Leadership in 2026
AI Without Trust Is AI Without ImpactAs AI becomes deeply embedded in enterprise decision-making, scrutiny is rising. Organizations no longer ask, “Can AI generate insights?” The real question is: “Can we trust those insights enough to act on them?”


